Background:
Poly ADP ribose polymerase inhibitors (PARPi) are widely used as maintenance treatment for ovarian cancer (OC) after response to platinum chemotherapy in the first line setting and recurrent disease. Their use has significantly improved outcomes, particularly in patients with BRCA mutations in whom first-line PARPi maintenance therapy increases progression-free survival from 13.8 to 56.0 months (Hazard Ratio, 0.33; 95% CI, 0.25-0.43). However, a recent meta-analysis of clinical trial adverse events and pharmacovigilance data showed that PARPi therapy significantly increases the risk of therapy related myeloid neoplasms (tMN), myelodysplastic syndrome (MDS) and acute myeloid leukaemia with poor outcomes. Platinum based chemotherapy is the mainstay of OC treatment and is associated with clonal haematopoiesis (CH) with mutations in DNA damage response genes. CH is recognised as a premalignant myeloid state but real world data on clonal dynamics during PARPi therapy are lacking. We present a case series of OC patients receiving PARPi-therapy referred for haematological review for beyond-expected HT or unexplained full blood count (FBC) abnormalities with serial FBC and myeloid next generation sequencing (mNGS) clonal monitoring in relation to PARPi therapy.
Methods:
Seven patients with stage III/IV high-grade OC were referred to haematology at the discretion of the treating oncologist between February 2017 and May 2022 (mean age (SD): 68 (7.0) years, median PARPi duration: 18 months (range: 4-86)). All patients had received prior platinum-based chemotherapy and were screened with mNGS in peripheral blood (PB) or bone marrow aspirate and trephine (BMAT) at initial assessment.
Results:
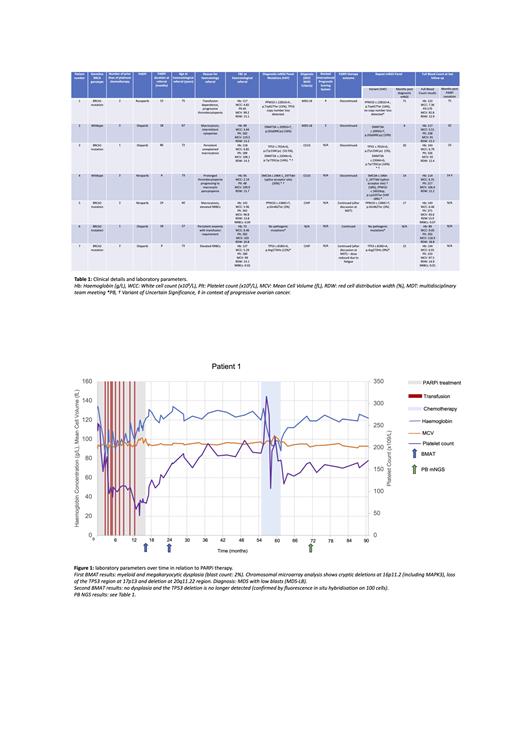
Reasons for referral are detailed in Table 1 and include two patients with isolated elevated nucleated red blood cell (NRBC) counts. Six patients had clonal pathology (CP), five of whom had single-nucleotide variants typical of platinum-exposure defined by the involved gene or platinum-associated mutational signatures. Clinical details including BRCA-status, haematological diagnosis and mNGS results are detailed in Table 1. PARPi-therapy was discontinued in four patients with CP, with resolution or improvement of FBC and m-NGS abnormalities (variant allele frequencies (VAFs) and/or copy number variations (CNVs)) in three patients. These findings were most marked in Patient 1, referred with grade 3 thrombocytopenia and transfusion dependence and in whom PARPi cessation resulted in transfusion independence and resolution of morphological and cytogenetic abnormalities (clinical course including BMAT results and haematological parameters illustrated in Figure 1), and Patient 3, who demonstrated selective regression of a TP53 mutated clone on PARPi cessation. Two patients with germline BRCA mutations continued PARPi therapy with 3 monthly PB mNGS monitoring after discussion at multidisciplinary team meetings.
Conclusions:
We show using sequential molecular and haematological monitoring before and after PARPi cessation that morphological, FBC and clonal abnormalities can be reversed on PARPi discontinuation, potentially mitigating the risk of life-threatening tMNs in some patients. We also highlight that, in addition to cytopenias, persistently elevated NRBCs may be an indicator of clinically relevant CP not well described in pharmacovigilance data based on common terminology criteria for adverse events criteria. Large scale prospective data on clonal dynamics in PARPi therapy are needed to inform mNGS screening and tMN risk stratification as scoring systems based on age associated clonal haematopoiesis of indeterminate potential may not be applicable. Until these data are available we demonstrate the value in MDT discussion and serial PB mNGS monitoring in formulating case-by-case therapeutic decisions regarding CP and PARPi therapy, particularly patients with high risk BRCA mutated OC.
Disclosures
Miller:MSD: Consultancy, Research Funding; GSK: Consultancy, Other: Travel grants, Speakers Bureau; AZD: Consultancy, Other: Travel grants, Speakers Bureau; Ellipses: Consultancy; Shionogi: Consultancy; Clovis Oncology: Consultancy, Speakers Bureau; Roche: Speakers Bureau. Lederman:Eisai: Membership on an entity's Board of Directors or advisory committees, Other: Lecture fees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Other: Lecture fees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Other: Lecture fees, Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Neopharm: Other: Lecture fees; Regeneron: Other: Steering /Data Monitoring Committee fees; Clovis Oncology: Other: Lecture fees; Pfizer: Other: Steering /Data Monitoring Committee fees; Nuvation: Membership on an entity's Board of Directors or advisory committees; Merck Sharp & Dohme Corp: Membership on an entity's Board of Directors or advisory committees, Other: lecture fees, Research Funding; Artios Pharma: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal